Genetic and epigenetic regulation of neural cell fate specification.
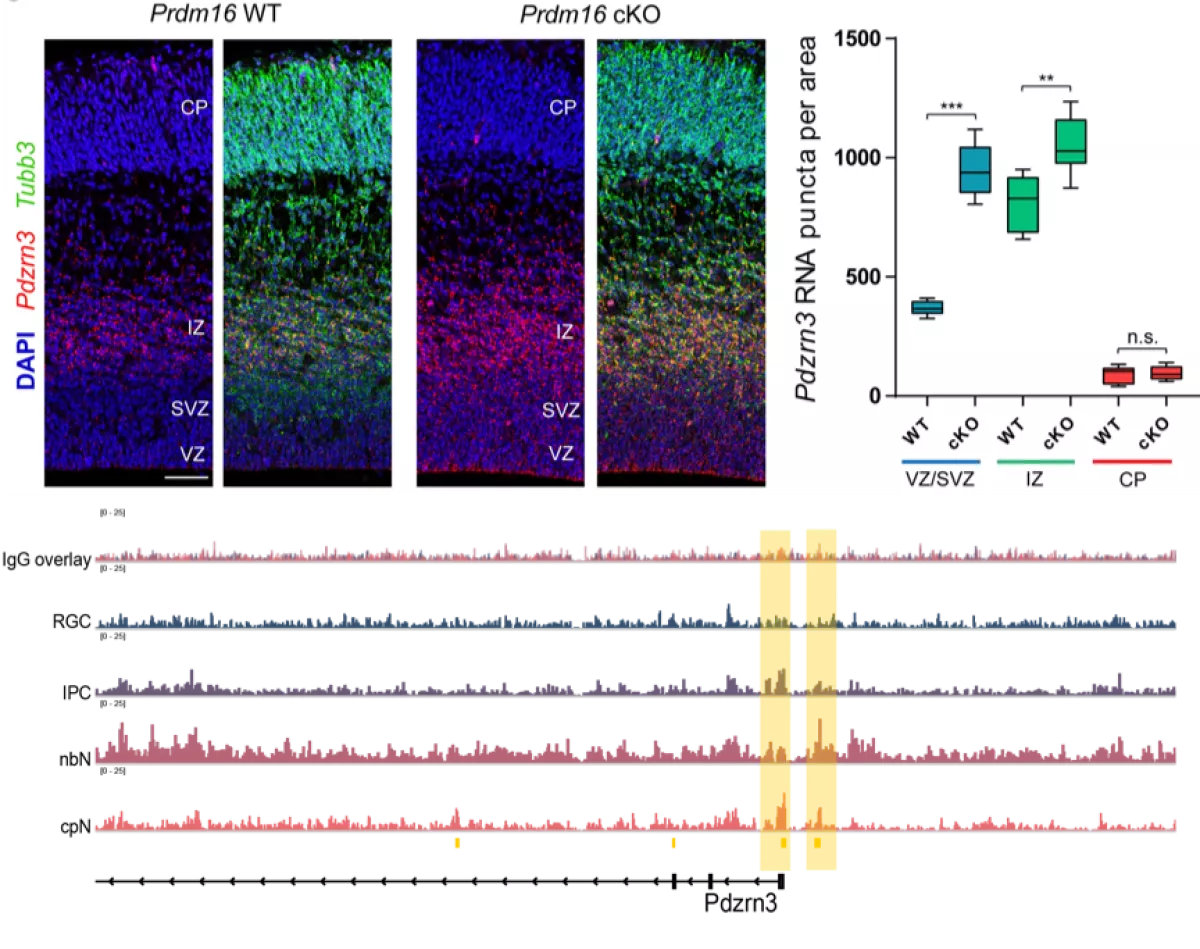
Radial glial cells serve as the primary neural progenitors of the central nervous system. Radial glial cells transition through a series of competence states as they undergo multiple divisions to produce clonal lineages composed of diverse neural cell types. We are interested in understanding how progenitors ‘know’ when to switch from producing one neural cell type to another and how epigenetic modifications in progenitors provide instructions to guide the fate specification of their neuronal progeny.
Developmental diversity of spatial and temporal lineages in the septum.
Septal nuclei in the basal forebrain have critical roles in regulating emotional and motivational states. The septum is composed of a diverse array of GABAergic, cholinergic and glutamatergic projection neurons. We know very little about the specific functions the diverse groups septal neuron types and even less about the developmental mechanisms that create this diversity. Our research is geared toward understanding the molecular programs involved in specifying these distinct projection neuron subgroups, and how their circuitry and functional properties regulates innate behaviors.
Neuron-glia crosstalk during neural circuit assembly
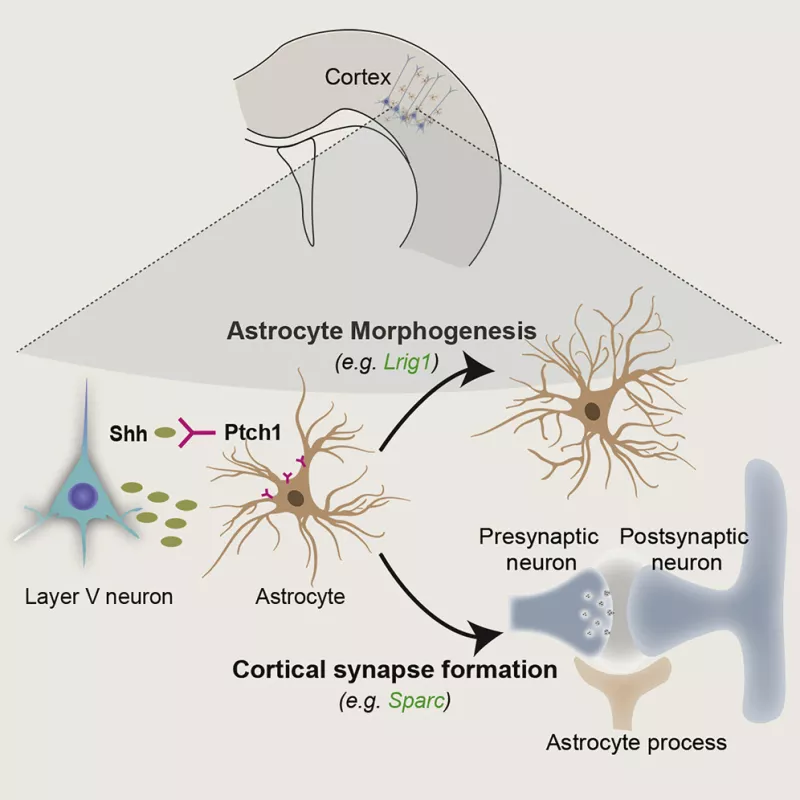
The nervous system is comprised of two main cell types, neurons and glia. Neurons connected through chemical and electrical synapses form complex networks in the brain. Glial cells have a broad range of functions that influence the formation and activity of neuronal networks. Astrocytes are the most numerous and diverse glial cell in the mammalian brain. They have crucial roles in regulation synapse formation and pruning. The mechanism by which astrocytes and neurons communicate and coordinate with is not well understood. We are interested in uncovering the secreted signals that neurons and astrocytes use to communicate, and the specific ‘messages’ encoded by those factors.